Garry Myers:
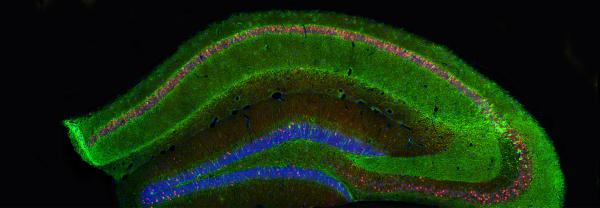
We are the Australian Institute for Microbiology and Infection, also called AIMI. We're a microbiology focused institute within the University of Technology, Sydney, within the Faculty of Science. The AIMI mission specifically is to decipher the interlinked problems of microbiology between humans, animals, and the environment. We are particularly interested in things such as antibiotic resistance. We're interested in developing new vaccines and therapeutics for antibiotic resistance in the first instance, but against a variety of animal diseases which have significant potential for economic impact.
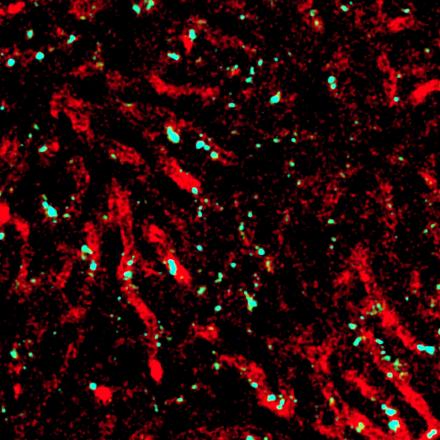
So AIMI has six research themes, they are quite broad based, all centering on the concept of microbiology, bacterial genomics, particularly where we sequence the complete catalog of genes. Transcriptomics, looking at what genes are turned on at any one point in time. Proteomics, where we try to catalog the complete complement of proteins. We are also making efforts to build expertise in the concept of bacterial epitranscriptomics, where we're looking at how RNA molecules are modified and how they use these modifications to avoid the immune system and avoid being attacked by the immune system.
AIMI has been funded by the Office of the Chief Scientist and Engineer in New South Wales to set up what's called the VRDC, which is the Vaccine and RNA Design Center, where we will be plugging into the major RNA investments that the New South Wales government is making for the manufacturing of injectable RNAs. Our focus will be on the development of vaccines and other therapeutics such as programmable antibiotics that are relevant for infection and disease.
AusGEM is a relationship that we've spent 10 years with New South Wales' Department of Primary Industries. This is an initiative to connect UTS's expertise and AIMI's expertise in genome scale biology with the biosecurity and animal plant mission of New South Wales DPI.
The challenge for any research institute is to identify those things that actually are going to make social benefit. Like vaccines, the antibiotics that we have now have taken decades to design and produce. Now that we have this capacity to rapidly generate vaccines, by taking advantage of an RNA ecosystem that's being built in South Wales in particular, but more broadly in Australia, we now have that translational angle where we are able to take our research, produce vaccines, produce RNA therapeutics much more quickly.
 Our people
Our people
 News and events
News and events Partner with us
Partner with us